missing translation for 'onlineSavingsMsg'
Learn More
Learn More
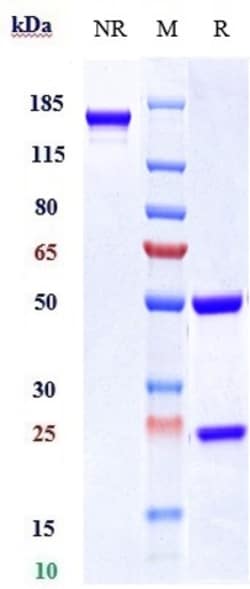
Invitrogen™ Tildrakizumab Recombinant Monoclonal Antibody


Descripción
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
Tildrakizumab is a high-affinity, humanized, IgG1 kappa antibody targeting interleukin 23 p19 that shows promise in the evolution of treatment strategy in chronic plaque psoriasis 3. The Food and pharmaceutical Administration (FDA) approved ILUMYA (tildrakizumab-asmn) for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy in March 2018. The approved recommended dosage of ILUMYA is a subcutaneous injection of 100 mg at Weeks 0, 4, and every 12 weeks thereafter 7. A study was performed on the pharmacokinetics of this pharmaceutical on various ethnicities. The pharmacokinetics of tildrakizumab were similar in Japanese, Caucasian, and Chinese subjects 5.

Especificaciones
Especificaciones
| Antígeno | Tildrakizumab Humanized |
| Aplicaciones | ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance |
| Clasificación | Recombinant Monoclonal |
| Concentración | 1 mg/mL |
| Conjugado | Unconjugated |
| Formulación | 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 |
| Alias de gen | SCH900222; MK-3222 |
| Especie del huésped | Human |
| Método de purificación | Protein A |
| Cantidad | 100 μg |
| Mostrar más |
Título del producto
Al hacer clic en Enviar, acepta que Fisher Scientific se ponga en contacto con usted en relación con los comentarios que ha proporcionado en este formulario. No compartiremos su información para ningún otro fin. Toda la información de contacto proporcionada se mantendrá de acuerdo con nuestra Política de Privacidad. Política de privacidad.
¿Detecta una oportunidad de mejora?