missing translation for 'onlineSavingsMsg'
Learn More
Learn More
Invitrogen™ Secukinumab Recombinant Monoclonal Antibody
Descripción
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
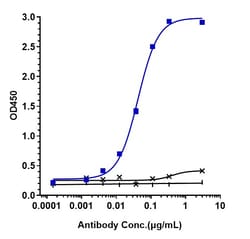
Secukinumab (Cosentyx) is a human monoclonal antibody designed for the treatment of uveitis, rheumatoid arthritis, ankylosing spondylitis, and psoriasis. Secukinumab is an interleukin-17A (IL-17A) inhibitor marketed by Novartis. IL-17 is a group of proinflammatory cytokines released by cells of the immune system and exist in higher levels in many immune conditions associated with chronic inflammation. By targeting IL-17A, secukinumab has shown excellent efficacy in psoriasis by normalizing skin histology and was approved by the United States Food and pharmaceutical Administration on January 21, 2015 to treat adults with moderate-to-severe plaque psoriasis.

Especificaciones
Especificaciones
| Antígeno | Secukinumab |
| Aplicaciones | ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance |
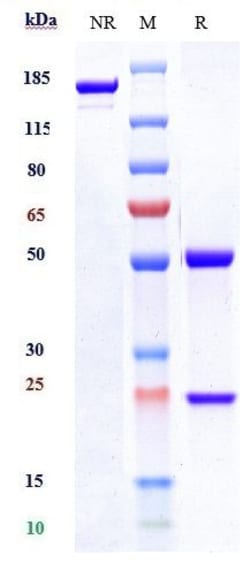
| Clasificación | Recombinant Monoclonal |
| Concentración | 1 mg/mL |
| Conjugado | Unconjugated |
| Formulación | 25mM Histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 |
| Alias de gen | AIN457; Cosentyx |
| Especie del huésped | Human |
| Método de purificación | Protein A |
| Cantidad | 1 mg |
| Mostrar más |
Título del producto
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.
Spot an opportunity for improvement?