missing translation for 'onlineSavingsMsg'
Learn More
Learn More
Invitrogen™ Dinutuximab Recombinant Monoclonal Antibody


Descripción
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
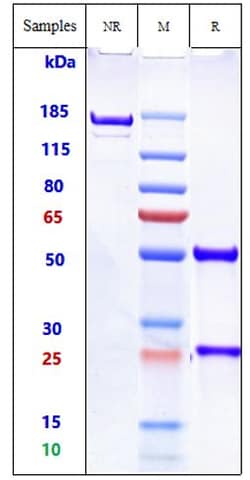
Dinutuximab is an IgG1 monoclonal human/mouse chimeric antibody against GD2, a disialoganglioside expressed on tumors of neuroectodermal origin, including human neuroblastoma and melanoma, with highly restricted expression on normal tissues. It is composed of the variable heavy- and light-chain regions of the murine anti-GD2 mAb 14. 18 and the constant regions of human IgG1 heavy-chain and kappa light-chain. By binding to GD2, dinutiximab induces antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity of tumor cells thereby leading to apoptosis and inhibiting proliferation of the tumor. It is indicated, in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2 (IL-2), and 13-cis-retinoic acid (RA), for the treatment of pediatric patients with high-risk neuroblastoma who achieve at least a partial response to prior first-line multiagent, multimodality therapy. Despite a high clinical response seen after first-line treatment, the complete eradication of neuroblastoma is rarely achieved and the majority of patients with advanced disease suffer a relapse. Current strategies for treatment include immunotherapy with pharmaceuticals such as dinutuximab to target surviving neuroblastoma cells and to prevent relapse.

Especificaciones
Especificaciones
| Antígeno | Dinutuximab Chimeric |
| Aplicaciones | ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance |
| Clasificación | Recombinant Monoclonal |
| Concentración | 1 mg/mL |
| Conjugado | Unconjugated |
| Formulación | 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 |
| Alias de gen | MAb-14.18; MOAB Ch14.18 |
| Especie del huésped | Human |
| Método de purificación | Protein A |
| Cantidad | 100 μg |
| Mostrar más |
Título del producto
Al hacer clic en Enviar, acepta que Fisher Scientific se ponga en contacto con usted en relación con los comentarios que ha proporcionado en este formulario. No compartiremos su información para ningún otro fin. Toda la información de contacto proporcionada se mantendrá de acuerdo con nuestra Política de Privacidad. Política de privacidad.
¿Detecta una oportunidad de mejora?